Bohr Model
Bohr atomic model, description of the structure of atoms, especially that of hydrogen, proposed (1913) by the Danish physicist Niels Bohr. The Bohr model of the atom, a radical departure from earlier, classical descriptions, was the first that incorporated quantum theory and was the predecessor of wholly quantum-mechanical models.
 The Bohr model and all of its successors describe the properties of atomic electrons in terms of a set of allowed (possible) values. Atoms absorb or emit radiation only when the electrons abruptly jump between allowed, or stationary, states.Immediately before 1913, an atom was thought of as consisting of a tiny positively charged heavy core, called a nucleus, surrounded by light, planetary negative electrons revolving in circular orbits of arbitrary radii.
The Bohr model and all of its successors describe the properties of atomic electrons in terms of a set of allowed (possible) values. Atoms absorb or emit radiation only when the electrons abruptly jump between allowed, or stationary, states.Immediately before 1913, an atom was thought of as consisting of a tiny positively charged heavy core, called a nucleus, surrounded by light, planetary negative electrons revolving in circular orbits of arbitrary radii.
Bohr amended that view of the motion of the planetary electrons to bring the model in line with the regular patterns (spectral series) of light emitted by real hydrogen atoms. By limiting the orbiting electrons to a series of circular orbits having discrete radii, Bohr could account for the series of discrete wavelengths in the emission spectrum of hydrogen. Light, he proposed, radiated from hydrogen atoms only when an electron made a transition from an outer orbit to one closer to the nucleus. The energy lost by the electron in the abrupt transition is precisely the same as the energy of the quantum of emitted light. Like Einstein's theory of the Photoelectric effect, Bohr's formula assumes that during a quantum jump a discrete amount of energy is radiated. However, unlike Einstein, Bohr stuck to the classical Maxwell theory of the electromagnetic field. Quantization of the electromagnetic field was explained by the discreteness of the atomic energy levels; Bohr did not believe in the existence of photons.
| Electrons can only gain and lose energy by jumping from one allowed orbit to another, absorbing or emitting electromagnetic radiation with a frequency ν determined by the energy difference of the levels according to the Planck relation |
Light described in modern physics
The modern theory of quantum mechanics came to picture light as (in some sense) both a particle and a wave, and (in another sense), as a phenomenon which is neither a particle nor a wave (which actually are macroscopic phenomena, such as baseballs or ocean waves). Instead, modern physics sees light as something that can be described sometimes with mathematics appropriate to one type of macroscopic metaphor (particles), and sometimes another macroscopic metaphor (water waves), but is actually something that cannot be fully imagined.
Visible light
 Visible light is the small part within the electromagnetic spectrum that human eyes are sensitive to and can detect. Visible light waves consist of different wavelengths. The colour of visible light depends on its wavelength. These wavelengths range from 700 nm at the red end of the spectrum to 400 nm at the violet end. White light is actually made of all of the colours of the rainbow because it contains all wavelengths, and it is described as polychromatic light. Light from a torch or the Sun is a good example of this. Light from a laser is monochromatic, which means it only produces one colour.
Visible light is the small part within the electromagnetic spectrum that human eyes are sensitive to and can detect. Visible light waves consist of different wavelengths. The colour of visible light depends on its wavelength. These wavelengths range from 700 nm at the red end of the spectrum to 400 nm at the violet end. White light is actually made of all of the colours of the rainbow because it contains all wavelengths, and it is described as polychromatic light. Light from a torch or the Sun is a good example of this. Light from a laser is monochromatic, which means it only produces one colour.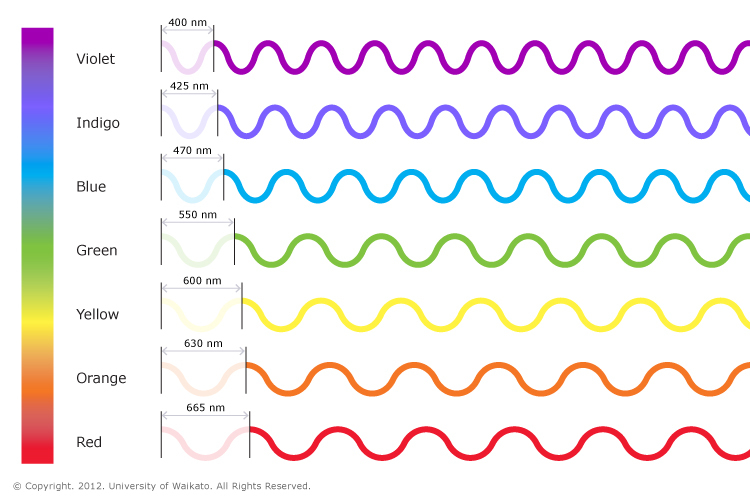 |
| The visible spectrum showing the wavelengths of each of the component colours. The spectrum ranges from dark red at 700 nm to violet at 400 nm.
Antimatter light spectrum
Atoms consist of electrons orbiting a nucleus. When the electrons move from one orbit to another they absorb or emit light at specific wavelengths, forming the atom's spectrum. Each element has a unique spectrum. As a result, spectroscopy is a commonly used tool in many areas of physics, astronomy and chemistry. It helps to characterise atoms and molecules and their internal states. For example, in astrophysics, analysing the light spectrum of remote stars allows scientists to determine their composition. With its single proton and single electron, hydrogen is the most abundant, simple and well-understood atom in the Universe. Its spectrum has been measured to very high precision. Antihydrogen atoms, on the other hand are poorly understood. Because the universe appears to consist entirely of matter, the constituents of antihydrogen atoms – antiprotons and positrons – have to be produced and assembled into atoms before the antihydrogen spectrum can be measured. It’s a painstaking process, but well worth the effort since any measurable difference between the spectra of hydrogen and antihydrogen would break basic principles of physics and possibly help understand the puzzle of the matter-antimatter imbalance in the universe.
ALPHA result is the first observation of a spectral line in an antihydrogen atom, allowing the light spectrum of matter and antimatter to be compared for the first time. Within experimental limits, the result shows no difference compared to the equivalent spectral line in hydrogen. This is consistent with the Standard Model of particle physics, the theory that best describes particles and the forces at work between them, which predicts that hydrogen and antihydrogen should have identical spectroscopic characteristics.
ALPHA is a unique experiment at CERN’s Antiproton Decelerator facility, able to produce antihydrogen atoms and hold them in a specially-designed magnetic trap, manipulating antiatoms a few at a time. Trapping antihydrogen atoms allows them to be studied using lasers or other radiation sources. The ALPHA collaboration reports the first ever measurement on the optical spectrum of an antimatter atom.
|
By Giannis Simantirakis Reczko
Physicist
Physics Department, University of Athens
Physics Department, University of Athens
References: https://en.wikipedia.org/wiki/Bohr_model
https://en.wikipedia.org/wiki/Light#Quantum_theory
https://www.sciencelearn.org.nz/resources/47-colours-of-light
https://home.cern/about/updates/2016/12/alpha-observes-light-spectrum-antimatter-first-time

Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου